2 - Stratospheric ozone depletion

✏️ Esta explicación aún no está disponible en tu idioma, haz clic aquí para sugerir tu traducción o escribe un correo electrónico a fdn.memo@marc-antoinea.fr.
Depending on its location in the atmosphere (at low or high altitude), ozone does not have the same effects. You may have heard of “good” and “bad” ozone. The ozone present at high altitude, in the stratosphere (20 to 50 kms) forms “the ozone layer” which filters and protects us from ultraviolet solar rays. This is "good ozone" and what we are talking about in this map. On the other hand, at low altitude, in the troposphere, ozone is present in small quantities and represents an air pollutant, as well as a short-lived greenhouse gas ("bad" ozone). It is a so-called “secondary” pollutant because it is not directly emitted by human activities but is formed by chemical reaction with other pollutants.
1Causa
Stratospheric ozone (which constitutes the ozone layer in the upper layers of the atmosphere between 20 and 40 km altitude), represents 90% of the ozone present in the atmosphere, and filters 97% of UVC rays . It is formed by the combination of a molecule of dioxygen (O2) with an isolated atom of oxygen (it is solar radiation which creates these isolated atoms by breaking the oxygen molecules in two). Chlorinated gases (like CFCs, used in particular as propellant gas in aerosol bombs) which reach the stratosphere are broken down by UV rays and release chlorine atoms which will react with ozone, transforming it into dioxygen.
Holes in the ozone layer were initially limited to Antarctica, before being observed on a smaller scale at the North Pole. Today, New Zealand and southern Australia, but also Canada and Scandinavia, regularly experience strong drops in stratospheric ozone in spring. Meteorological conditions are different between the South Pole and the North Pole, which could explain why the phenomenon is more marked in Antarctica (south) than in the Arctic (north). Particularly because there is more renewal of air masses at the north pole, while in the southern hemisphere, air currents "isolate" the air masses, resulting in less mixing, and therefore no ozone contribution from equatorial regions.
Governments decided to end the production of CFCs within the framework of the Montreal Protocol which came into force on January 1, 1989. They were replaced initially by HCFCs (which are less destructive of ozone), then by HFCs in a second step (they do not contain chlorine, therefore will not react with ozone).
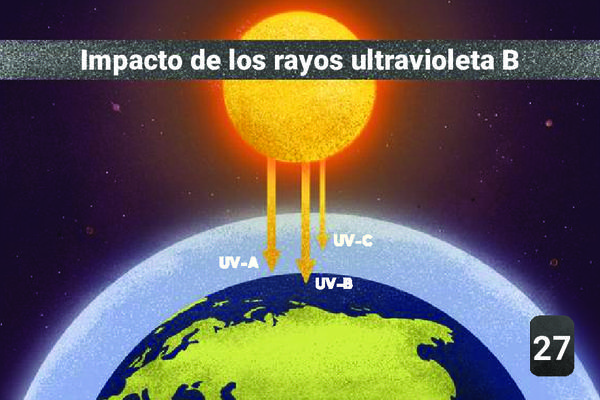
1Consecuencia
The UVA/UVB/UVC distinction is due to a different wavelength range. UVC never reaches the earth's surface, it is entirely filtered by the atmosphere. UVB and UVA partially pass through the atmosphere depending on the concentration of nitrogen, oxygen, oxygen and ozone. The latter is the most influential on UVB filtration.
1Otra consecuencia posible
The ozone layer -> climate change link can be true or false depending on the justification given. The ozone layer filters part of UV rays, therefore helping to limit the energy reaching the earth's surface. However, this impact is not significant. This link is therefore true, even if secondary. It is likely that most of the participants making this link do so with a poor justification in mind (confusion of the ozone layer and climate change phenomena). It is therefore important to check what they have in mind with this link. During pollution peaks, ozone is produced at low altitude, which is a greenhouse gas and heats up locally. On the other hand, this ozone being produced at low altitude, it is not part of the "ozone layer", and therefore does not justify an ozone layer -> climate change link.
2Causas incorrectas
Aerosols are small particles suspended in the air (fine particles, soot from volcanoes, etc.) and have no impact on the ozone layer. They should not be confused with aerosol bombs which used chlorine gases (CFCs), which were responsible for the hole in the ozone layer.
CO2 is not responsible for the destruction of O3 ozone molecules, it is chlorine molecules (which are contained in CFC chlorine gases).